- Home
- Share
- Forum
- Sleep apnea Forum
- Living with sleep apnea
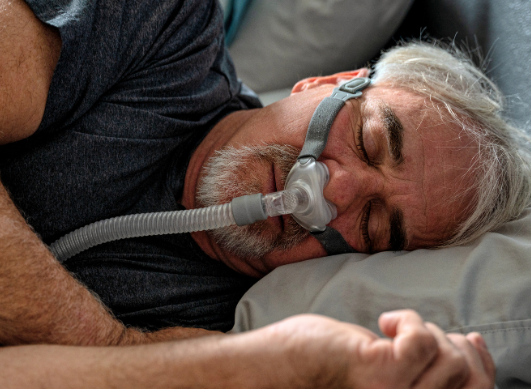
- Phillips recall on CPAP machines: Are you affected?
Patients Sleep apnea
Phillips recall on CPAP machines: Are you affected?
- 187 views
- 3 times supported
- 16 comments
All comments
Go to the last comment

Courtney_J
Community managerGood advisor
![]()
Courtney_J
Community manager
Last activity on 08/08/2022 at 11:09 AM
Joined in 2020
1,340 comments posted | 38 in the Sleep apnea Forum
6 of their responses were helpful to members
Rewards
-
Good Advisor
-
Contributor
-
Messenger
-
Explorer
-
Friend
Hello @Bsavidge, thank you for opening this discussion! Do you have one of the recalled machines? Has your doctor recommended that you stop using it while Philips works on repairing or replacing it? Let me tag some other members who may be interested in this.
For anyone who would like more information about the recall, including possible steps to take, you can consult the FDA communication page here: Certain Philips Respironics Ventilators, BiPAP, and CPAP Machines Recalled Due to Potential Health Risks: FDA Safety Communication
Hi everyone, are you aware of the recall on certain Philips CPAP and BiPAP devices and ventilators? Is your device included in the recall? Have you been in touch with your doctor about it? If so, what does he/she say? Have you been in touch with Philips?
@jqlyons @mlleelise @Amostayar @ggudanowski @NoFilter62 @Momov3burros @Full1234 @Divegal56 @nu2rasln @Nisey69 @Lorikat81 @Tilly77 @CharlotteB @Itwerks @ncbonner @RalphB @bturcotte @Ronda01 @PaulC53
Take care,
Courtney
See the signature
Courtney_J, Community Manager, Carenity US
![]()
Bsavidge
![]()
Bsavidge
Last activity on 05/15/2022 at 11:44 AM
Joined in 2020
2 comments posted | 2 in the Sleep apnea Forum
Rewards
-
Explorer
Hello Courtney,
It is a mess from Phillips. They said it could take a year and they are still waiting for FDA approval. This is a joke. Those of us with sleep apnea can not wait for a year to either have the current machine replaced or fixed. This is ridiculous. I have been in touch with my Dr. and the advice was if the machine does not smell or you do not see debris particles in the water container then we should keep using it. This is not a fix for this issue.
Bob

Courtney_J
Community managerGood advisor
![]()
Courtney_J
Community manager
Last activity on 08/08/2022 at 11:09 AM
Joined in 2020
1,340 comments posted | 38 in the Sleep apnea Forum
6 of their responses were helpful to members
Rewards
-
Good Advisor
-
Contributor
-
Messenger
-
Explorer
-
Friend
@Bsavidge Oh gosh, that isn't a fix at all! With how serious sleep apnea can be, you'd hope that they would be more proactive in protecting and helping consumers.
For those of you who have Philips devices, here is a link to the Philips site where you can register your device with them and be put on a waitlist for a replacement: Philips Recall Registration. They also have a phone number you can call here: 877-907-7508.
See the signature
Courtney_J, Community Manager, Carenity US
![]()
rainbowgerl
![]()
rainbowgerl
Last activity on 07/13/2022 at 2:32 AM
Joined in 2020
12 comments posted | 9 in the Sleep apnea Forum
Rewards
-
Contributor
-
Explorer
-
Friend
-
Newsfeeder
Yes. I use a Phillips. Said up to.a year.
My doctor could not say continue to use. I decided to continue. Maybe not the best
Diane
See the signature
Diane
![]()
JeanMM
![]()
JeanMM
Last activity on 02/18/2023 at 4:20 PM
Joined in 2021
1 comment posted | 1 in the Sleep apnea Forum
Rewards
-
Explorer
My husband and I both use a clap. He because he stops breathing frequently. I use it because while I quit breathing it’s scary because my O2 levels go way down. I can smell hot wires bad enough that I was getting up and checking the walls around plug ins and switches and feeling for warm power cords. Haven’t smelled it since I quit using it. Our dr and Phillips said we had to make our own decision.
![]()
snoozer
![]()
snoozer
Last activity on 04/23/2023 at 4:51 AM
Joined in 2021
1 comment posted | 1 in the Sleep apnea Forum
Rewards
-
Explorer
I am new to this group. I also have a recalled C-pap machine. I have not used it since August. I was first advised by the sleep clinic not to use it. My PCP said to follow their advice. I just talked to the sleep clinic again and I was encouraged to use it again since my apnea is severe. i had pulmonary function tests done (due to shortness of breath) and that did not show any damage to my lungs. So I am going to go back on it. My C-pap has been registered since August. I miss the treatment, I have been on C-pap since 1994. It is a part of my life now.
Snoozer
![]()
Python287
![]()
Python287
Last activity on 12/17/2022 at 6:33 PM
Joined in 2020
12 comments posted | 12 in the Sleep apnea Forum
Rewards
-
Contributor
-
Explorer
I started using a RESMED Airsense 10 that I purchased some time ago for trips. I put my Dreamstation in a box and now just waiting ro Phillips to get off their butts and replace my machine. I am not at all pleased with Phillips.

Gordon
Good advisor
![]()
Gordon
Last activity on 11/09/2024 at 5:12 PM
Joined in 2018
50 comments posted | 5 in the Sleep apnea Forum
7 of their responses were helpful to members
Rewards
-
Good Advisor
-
Contributor
-
Committed
-
Explorer
-
Evaluator
Courtney:
Thank you for including me in this discussion. I was diagnosed with moderate to severe Apnea about 2 years ago and prescribed the Philips Bi-PAP therapy machine. Within the first year of use, my kidney function went from over 80% down to about 30%, and I had three severe sinus infections. After the second sinus infection I purchased a case of in-line filters for the Bi-PAP machine and started using them once the recall was announced in June. In May I had sinus surgery to clear an acute blockage but then in late October was prescribed another round of antibiotics for the fourth sinus infection. My next sinus CT Scan is scheduled for next week. Also having COPD/Emphysema for about 15 years, after my sinus surgery I had a recent acute exacerbation that required prednisone and I am now on daily Albuterol Nebulizer treatment as well as the other prescribed daily inhalers.
As soon as I was made aware of the recall and registered with Philips, I checked with the DME, Medicare and my insurance provider, Humana. The DME told me that my machine would be replaced with a new Script. Medicare and Humana both said that the cost of the replacement would be covered at 100%. Soon thereafter the DME changed their mind and has declined to replace the machine to date, telling me that I need to go directly through Philips. As you probably know. Philips has about 4 million devices in the US to repair or replace and they anticipate up to a year to complete.
Philips has been aware of this issue of the recall (sound abatement foam) for a number of years. They recently came out with the DreamStation 2 for C-PAP users with different foam. That foam had tentative approval from the FDA. Fixes for the Bi-PAP machines are more involved and they just started sending out some refurbished machines with the "approved" foam. Now the FDA inspected the Philips plant, their records and the replacement foam to find out it may be just as deadly as the original foam.
I have lots more information on this since I not only have Apnea but several chronic conditions that affect my health, I record and save information relating to them all. I also have had some arguments with others regarding the use of the bacterial filters and their efficiency on the Bi-PAP machine but will offer the attached image of a new filter along side one only in use for 5 to 7 days. This is in a machine that is nearly 2 years old...
I am convinced that a percent of my current health issues are directly related to the foam used in the Philips machines, but I also understand that Philips, or any company, will take some time to sort it all out when you consider the huge number of devices affected. When adding in ventilators to the numbers of PAP machines worldwide, you are talking 12 to 15 million devices.

See the signature
Gordon Harvey

Guygraff
![]()
Guygraff
Last activity on 11/08/2024 at 12:42 PM
Joined in 2020
9 comments posted | 4 in the Sleep apnea Forum
Rewards
-
Contributor
-
Explorer
I have a recalled machine. They (philips) asked me "how I cleaned it," my reply was "mild soap and warm water as per my initial instructions".
They then asked if I used ozone or harsh chemicals to clean it. My answer "no"
They're comment was "it is safe to use until I receive the replacement cpap"
See the signature
Guy Graff

Gordon
Good advisor
![]()
Gordon
Last activity on 11/09/2024 at 5:12 PM
Joined in 2018
50 comments posted | 5 in the Sleep apnea Forum
7 of their responses were helpful to members
Rewards
-
Good Advisor
-
Contributor
-
Committed
-
Explorer
-
Evaluator
Ozone is not the cause of the recall. Foam that off-gasses VOCs and emits particles from day one are the causes of the recall. Ozone may exacerbate the deterioration of the foam, but the foam with or without the introduction of ozone will deteriorate and off-gas.
I also find it hard to believe that Philips would say that any device under recall was safe to use until a replacement is received. Philips has been very careful in their wording and has left it up to doctors to decide what is safe or not.
See the signature
Gordon Harvey
Give your opinion
Members are also commenting on...
Articles to discover...

12/12/2018 | Testimonial
Fighting Schizophrenia Symptoms: a Long Journey Against Paranoia after Denial and being Admitted
Subscribe
You wish to be notified of new comments
You have been subscribed







Bsavidge
Bsavidge
Last activity on 05/15/2022 at 11:44 AM
Joined in 2020
2 comments posted | 2 in the Sleep apnea Forum
Rewards
Explorer
Why is it taking so long with Phillips either fixing or replacing the recalled machines that are linked to cancer. This is truly pathetic the information that is being presented to the people who have the recalled machines.