HUMIRA (Adalimumab): We tell you everything!
Published Jan 25, 2021 • By Doriany Samair
HUMIRA® is widely used for several health conditions.
What is adalimumab? How does it work and what are the differences with other TNF inhibitors? What precautions should be taken or what are the contraindications? What are the adverse effects?
Let us tell you everything in this article!

What is HUMIRA® or Adalimumab?
A TNF-alpha inhibitor monoclonal antibody
Adalimumab, sold under the brand name HUMIRA®, is a type of biotherapy. More specifically, it is a human monoclonal antibody (as indicated by the suffix -umab: "u" for "human" and "man" for "monoclonal antibody") which targets the pro-inflammatory cytokine TNF-alpha, also called "Tumor Necrosis Factor" or "tumor necrosis factor alpha". These monoclonal antibodies are made by cloning white blood cells in a laboratory.
TNF-alpha is released by the body in response to infection or to the presences of tumor cells: it is a true inflammation mediator involved in the immune defense system.
However, in chronic inflammatory conditions, immune dysfunction causes TNF-alpha to persist at too high levels, which has harmful consequences on the body's tissues in the long term. Indeed, it is a mediator that is highly involved in the physiopathology of chronic inflammatory diseases.
This is why, as part of the treatment of inflammatory diseases, the aim is to neutralize excess TNF-alpha. Adalimumab is able to bind specifically to TNF-alpha proteins and thus diminish the inflammatory process.
How is it different than other TNF inhibitors?
Other monoclonal antibodies
Derived from the same therapeutic class, the other anti-TNF alpha monoclonal antibodies have a different composition: some are humanized, or come mostly from a human source, such as certolizumab (CIMZIA®; suffix -Zu-mab, "zu" meaning "humanized"), some come from murine cells (and are used rarely today due to their tendency to cause allergic reactions in humans), and others are chimeric, having both human and murine origins, such as infliximab (REMICADE®; suffix -Xi-mab, "xi" meaning "chimeric").
Similarly to adalimumab, golimumab (SIMPONI®) is also is a fully human monoclonal antibody.
The half-life of these antibodies can vary from one antibody to another, meaning that some can survive longer than others in the body.
Etanercept
There are other substances such as etanercept (ENBREL®) which have TNF-alpha inhibiting properties without being a monoclonal antibody: it is a soluble TNF-alpha receptor, capable of competitively inhibiting the binding of TNF-alpha with its surface cell receptors. As a result, the biological activity of TNF-alpha is blocked.
Indications, precautions for use and contraindications
Conditions treated by HUMIRA®
- Moderately to severely active rheumatoid arthritis in adults after failed disease-modifying therapies or methotrexate;
- Severe chronic plaque psoriasis in adults (psoriatic arthritis) ;
- Active, moderate-to-severe Crohn's disease in adults;
- Active, severe Crohn's disease in children and teenagers from the age of 6, as a second-line treatment;
- Ankylosing spondylitis;
- Active ulcerative colitis;
- Uveitis;
- Juvenile idiopathic arthritis;
- Hidradenitis suppurativa (Verneuil's disease).
Precautions for use and contraindications
It is worth remembering that HUMIRA® is injected under the skin. It comes as a pre-filled injectable pen and is kept in the refrigerator, away from light. It is to be taken out of the refrigerator 15 to 30 minutes before injection. There are a few exceptions to the storage conditions: when travelling, the pen can be stored at room temperature, up to 77°F, away from light, for up to 14 days.
During treatment with HUMIRA®, corticosteroids or other immunomodulators (drugs capable of modifying the immune response) must be taken under prior medical supervision, if they cannot be postponed.
There are a few situations in which administration of HUMIRA® is contraindicated:
- In case of allergy (hypersensitivity) to adalimumab or any other component of the product;
- In case of active tuberculosis, it is imperative to inform your doctor about the condition or recent contact with a tuberculosis patient. Tuberculosis can be facilitated by the immunosuppression associated with the drug. This is why it is important to remain vigilant at the first signs of infection such as a fever, even a slight fever, a persistent cough, fatigue or significant weight loss.
- In case of a moderate-to-severe heart failure, or other cardiac problems.
Other situations require increased monitoring and vigilance:
- In case of mild heart failure, close monitoring will be necessary to prevent the disease from worsening;
- In case of hepatitis B or risk of hepatitis B infection, the doctor must be informed as viral reactivation can be severe with immunosuppressive treatment;
- In case of multiple sclerosis other demyelinating diseases (a disease of the myelin sheath, which surrounds the neurons and destroys them), the doctor will determine the suitability of HUMIRA® for the patient;
- In the case of opportunistic infections, it is essential to ensure that the patient is not suffering from another infection, even if it is localized. HUMIRA® helps to reduce the body's resistance to infections and external aggressions. It is not uncommon for infections to develop more easily during treatment. It is therefore advisable to inform the doctor of any infectious symptoms (wounds, dental infection, fever and fatigue), especially in patients over 65. The doctor should also be aware of any planned travel to an endemic area for certain fungal, viral or parasitic infections.
- In case of underlying cancers;
- In case of COPD, treatment with HUMIRA® should be discussed;
- In case of pregnancy, HUMIRA® is not recommended. An effective method of contraception must be used during treatment and for at least 5 months after stopping the treatment. The use of this medicine in pregnant women increases the risk of the fetus developing a serious infection. Breast-feeding while on HUMIRA® is also not recommended.
Intake and adverse effects
HUMIRA ® is a pre-filled single-use pen to be injected under the skin:
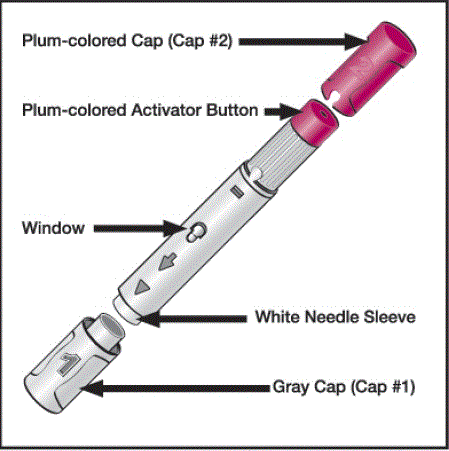 Figure 1. Pre-filled Humira pen
Figure 1. Pre-filled Humira pen
Source: RxList: Humira
The injection site should be at least 1 inch away from the previous location: on the thigh or abdomen (2 inches from the belly button). The injection site should be disinfected beforehand, clean and free of skin lesions or any particular sensitivity. Before following the injection instructions, the patient should make sure that the pen contains a clear and colorless liquid.
Adverse side effects
On average, Carenity members have given HUMIRA® a rating of 5.32 out of 10.
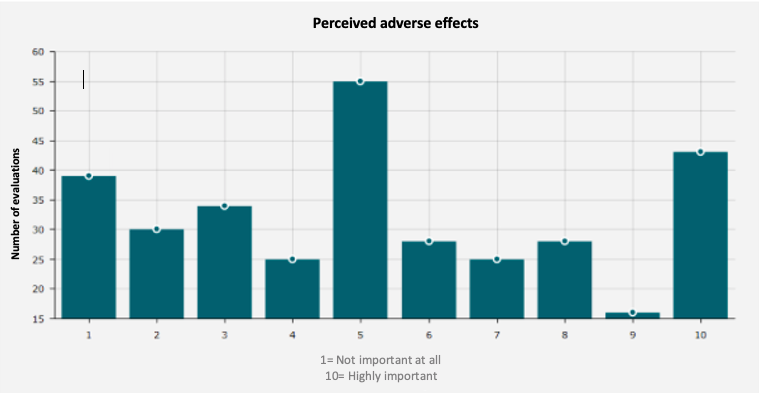
Figure 2. Adverse effects
Source: "Give my opinion: Humira" - Carenity
Indeed, HUMIRA® can lead to a number of adverse side effects which are often reported by patients. Despite the fact that they have helped to make significant advances in the care of chronic inflammatory diseases, TNF-alpha inhibitor biotherapies do not spare patients from limiting side effects, which can sometimes range from serious to severe. The most frequent side effects are include, but are not limited to:
- Respiratory tract infections (rhinopharyngitis, sinusitis, pneumonia)
- Headaches and migraines
- Abdominal and musculoskeletal pains
- Nausea and vomiting
- Skin rash
- Many types of infections have been reported (systemic, fungal, urinary, joint, skin, etc.)
- Sleep disturbances
- Anxiety and depression
- Metabolic disorders
It is possible to report any adverse reaction, even if it is not mentioned in the label or medicine instruction pamphlet, to the Food and Drug Administration's (FDA) medical product safety reporting program, MedWatch, in order to contribute to the safe use of this medicine.
Was this article helpful to you?
Share your thoughts and questions with the community in the comments below!
Take care!
Sources:
- "Les anti TNF alpha" - Pharmacomédicale
- "Adalimumab" - Vidal.fr
- "HUMIRA, avis de la commission de transparence 2017" - HAS
- "HUMIRA, avis 2012" - HAS
- https://www.has-sante.fr/upload/docs/application/pdf/2013-01/humira_fit_14122012.pdf
- "Humira, stylo 40 notice" Abbvie
- "Etanercept" - Vidal.fr
- "Give my opinion: Humira" - Carenity
Comments
You will also like

Spoon theory: What is it and how can it help people living with chronic illness?
Apr 13, 2022 • 8 comments

What is the psychological impact of chronic pain? Carenity members share their experience!
May 27, 2021 • 9 comments

 Facebook
Facebook Twitter
Twitter
