Vaccines against COVID-19 and its variants: What's the latest?
Published Mar 31, 2021 • By Clémence Arnaud
For the past year, the Sars-CoV-2 virus has been responsible for a global pandemic. Numerous vaccines have been developed to combat the virus with the aim of creating herd immunity. Virus mutations have also emerged in recent months, some of which are cause for concern.
What is a coronavirus variant exactly? Which vaccines are available and for whom? Do vaccines protect against all variants of COVID-19?
We tell you everything in our article!

Which vaccine for which age group?
The coronavirus vaccination campaign has started across the US, with 3 available vaccines, developed by the Pfizer-BioNTech®, Moderna®, and Johnson & Johnson® labs. A number of other vaccines are currently being trialed and/or are awaiting approval by the FDA, most notably the AstraZeneca® and Novavax® vaccines.
After concerns early in the year within the EU about potential risk of blood clots in younger recipients of the AstraZeneca® vaccine, the European Medicines Agency (EMA) temporarily suspended its use. After thorough examination, both the EMA and the UK Medicines & Healthcare products Regulatory Agency concluded that the vaccine was both effective and safe for use. The AstraZeneca® vaccine has not yet been approved by the FDA.
The CDC has provided recommendations for federal, state, and local governments about who should be vaccinated in priority, following Advisory Committee on Immunization Practices (ACIP) advice. State and local vaccination campaigns generally follow the phases listed in the following image*:
Source: Commonwealth of Massachusetts
*This is only an example from the state of Massachusetts, CDC guidelines for vaccine allocation are as follows:
- Phase 1a:
- Healthcare personnel
- Residents of long-term care facilities
- Phase 1b:
- Frontline essential workers - firefighters, police officers, corrections officers, food and agricultural works, USPS workers, manufacturing workers, grocery store workers, public transit workers, and those who work in the education sector (teachers, support staff, daycare workers
- People aged 75+ - People in this age group who are also residents of long-term care facilities should be offered vaccination in Phase 1a
- Phase 1c:
- People aged 65-74 - People in this age group who are also residents of long-term care facilities should be offered vaccination in Phase 1a
- People aged 16—64 years with underlying medical conditions which increase the risk of serious, life-threatening complications from COVID-19. You can find a list of underlying medical conditions the CDC considers to lead to an increased risk of severe illness from COVID-19 here: People with Certain Medical Conditions
- Other essential workers - those who work in transportation and logistics, food service, housing construction and finance, information technology, communications, energy, law, media, public safety, and public health.
While the CDC makes recommendations for who should be offered COVID-19 vaccine first, each state has its own plan for deciding who will be vaccinated first and how they can receive vaccines. Make sure to consult your local health department for more information on COVID-19 vaccination in your area.
Vaccination and specific cases
- Pregnant women: There is no known risk with giving inactivated virus or bacterial vaccines during pregnancy. However, there is still little data on the COVID vaccine in pregnant women, so it is recommended to consult a health care professional (doctor, midwife or gynecologist) to assess the situation and the benefit/risk of vaccination on a case by case basis.
If you are pregnant and have received a COVID-19 vaccine, the CDC encourages you to enroll in v-safe, the CDC’s smartphone-based tool that uses text messaging and web surveys to provide personalized health check-ins after vaccination. A v-safe pregnancy registry has been established to gather information on the health of pregnant people who have received a COVID-19 vaccine - People who have already had COVID-19: When infected by the coronavirus, patients develop a lasting immunity. However, it is still unclear how long that immunity lasts. Therefore, people who have already had COVID-19 should get vaccinated. There is no evidence of any safety concerns from vaccinating people who have had COVID in the past or who currently have the COVID-19 antibodies.
If you were treated for COVID-19 with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine. Talk to your doctor if you are unsure what treatments you received or if you have more questions about getting a COVID-19 vaccine. - People who are currently sick with COVID-19: People who currently have COVID symptoms should wait to get vaccinated until they have recovered from their illness and have met criteria to discontinue isolation. Those who have COVID but have no symptoms should also wait until they meet the criteria for ending quarantine to get vaccinated. This also applies to people who get COVID-19 before receiving their second dose of vaccine.
- People who have been in contact with someone who has COVID-19: People who have been in contact with someone who has tested positive for COVID-19 should wait for a negative COVID test result before receiving the vaccine.
Vaccines and the COVID-19 variants
The SARS-CoV-2 epidemic has been a global phenomenon for more than a year. The appearance of variants makes the fight against the virus even more complex. Indeed, it is important to detect these variants and to study them in order to be able to anticipate the potential consequences that they may have on the population.
A variant is an organism that differs from the original virus (in this case SARS-CoV-2) by several mutations. A mutation is the substitution of one RNA base (in the case of RNA viruses) by another during a replication error, which results in the modification of the corresponding amino acid on the protein coded by the mutated gene. This mutation can result in a substitution (one amino acid replaces another), a deletion (one amino acid is lost), an insertion (a new amino acid is introduced into the protein), a duplication (one amino acid is abnormally repeated), etc.
Not all variants of the COVID-19 virus are more virulent or more infectious than the original virus. The ones we will be looking out for in particular are the so-called "variants of concern" (VOC). These are either more contagious, more virulent or are capable of immune evasion (an ability to evade a host's immune response to either spread to a new host or to continue growing).
The three variants that we have been hearing a lot about are the following:
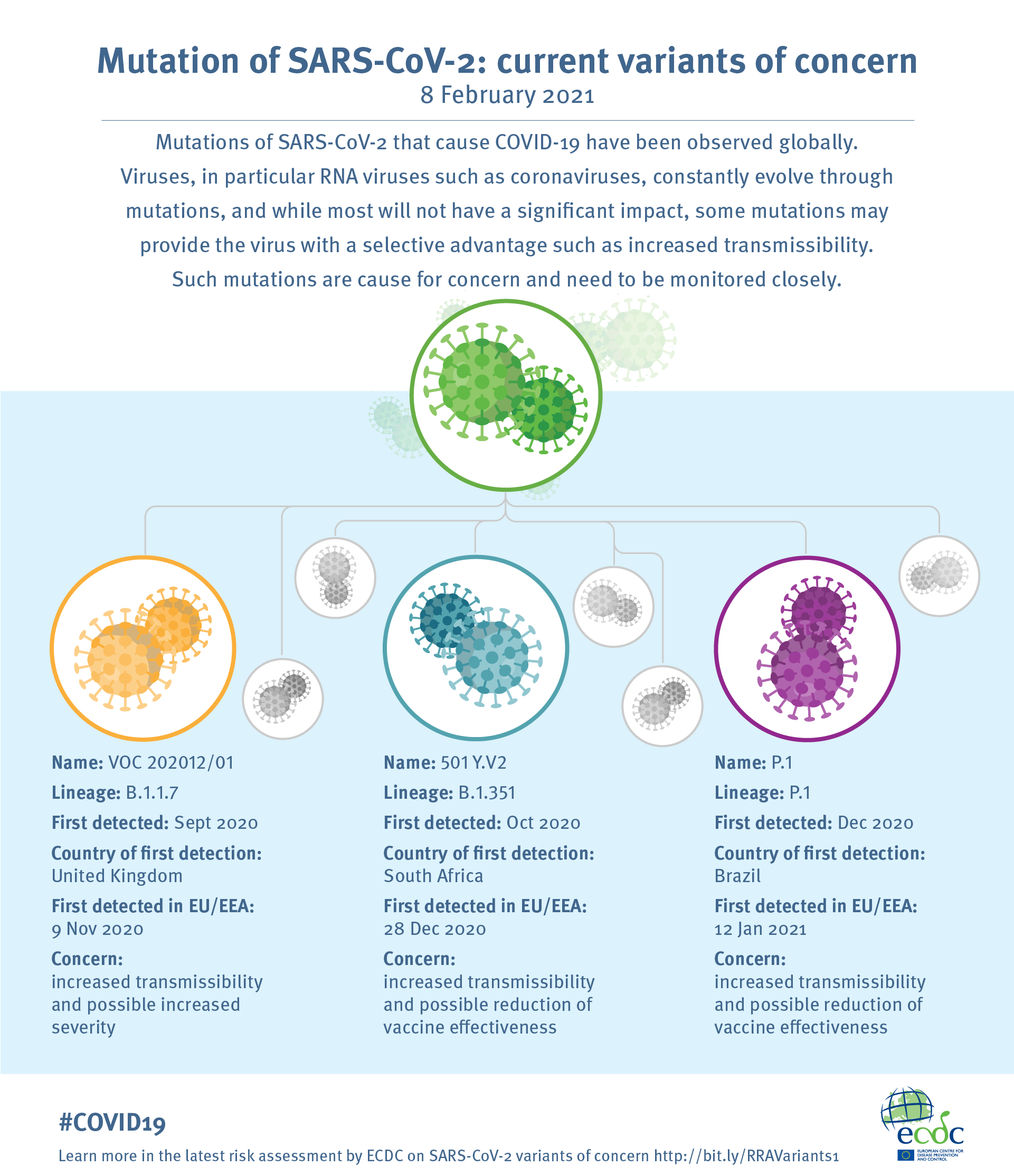
Source: European Centre for Disease Prevention and Control
- Lineage B.1.1.7, also known as VOC-20DEC-01 or the UK variant: This variant was first detected in and spread from South East England (Kent) and is defined by 23 mutations from the original coronavirus. This variant is 50-70% more transmissible than the original Sars-CoV-2, but is no more or less virulent. The main concern for this variant surrounds its mutations that impact the structure of the Spike protein, which allows the virus to enter and infect our cells. The Spike protein is also the target of the current vaccines on the market.
Additionally, this variant was initially not detected by certain PCR tests, resulting in false negatives. Since then, all commercial tests now detect the UK variant. - Lineage B.1.351, also known as 501 Y.V2 or the South African variant: This variant, first detected in the Eastern Cape province of South Africa, also has mutations in its Spike protein and is more transmissible than the original COVID variant. Cases of re-infection have also been seen with this variant. Initial data has shown that the AstraZeneca® vaccine does not appear to be sufficiently effective against this variant, but the Pfizer-BioNTech® and Moderna® messenger RNA vaccines have shown efficacy against it.
- Lineage P.1, also known as VOC-202101/02 or the Brazil(ian) variant: The mutations in this strain seem to give it increased transmissibility as well as increased resistance to immunity.
All three variants have been found in the US, the CDC has set up a helpful tool for tracking cases of each variant by state here.
A few new variants have also been found in the US, such as the B.1.526 in New York and the B.1.427 in California, but there is little information to date about the transmissibility and virulence of these variants. Studies on both are underway.
The emergence of these variants is prompting pharmaceutical companies to continue research into existing and future vaccines.
Have you been vaccinated against the coronavirus? What do you think of the available vaccines?
Was this article helpful to you?
Share your thoughts and questions with the community in the comments below!
Take care!
Sources:
- INSERM - Un variant du SarS-CoV-2 inquiétant, vraiment ?
- Vidal - COVID-19 : mutations, variants, lignées, N501Y, E484K... De quoi parle-t-on ?
- Different COVID-19 Vaccines, Centers for Disease Control and Prevention
- CDC’s COVID-19 Vaccine Rollout Recommendations, CDC
- Advisory Committee on Immunization Practices (ACIP), CDC
- About Variants of the Virus that Cause COVID-19, CDC
- Information about COVID-19 Vaccines for People who Are Pregnant or Breastfeeding, CDC
- Frequently Asked Questions about COVID-19 Vaccination
- Mutation of SARS-CoV2 - current variants of concern, European Centre for Disease Prevention and Control
Comments
You will also like

What are the dangers associated with the over-the-counter sale of certain medicines?
Dec 19, 2020 • 6 comments

 Facebook
Facebook Twitter
Twitter

