COVID-19 vaccines: Information and opinions of patients with chronic illness
Published Jan 30, 2021 • By Clémence Arnaud
The COVID-19 pandemic has been sweeping the globe for more than a year, with more than 2 million people now dead from the virus. Vaccine development and commercialisation are now the focus of attention.
What is a vaccine? What are the stages of vaccine development? What are the differences between the different vaccines for COVID-19? Why is vaccination a strategy to fight COVID-19? What do patients with chronic illnesses think?
We tell you all about it in our article!
Everything to know about vaccines:
What is a vaccine? Definition:
According to the WHO, the definition of a vaccine is as follows: "A vaccine is a preparation administered to induce immunity to a disease by stimulating the production of antibodies. Vaccines include suspensions of inactivated or attenuated microorganisms, or products or derivatives of microorganisms".
Different types of vaccines:
- Live attenuated vaccines: These are developed using viruses or bacteria that have been modified to make them less aggressive.
- Inactivated vaccines: They do not contain the pathogenic agent, but they may contain:
- A fragment of the pathogen (hepatitis B, tetanus).
- The totally inactivated pathogen (whooping cough)
- A small part of the pathogen, a protein or nucleic acid (RNA or DNA).
Vaccine composition:
All vaccines are made up of a vaccine antigen that corresponds to the active substance of the vaccine. The adjuvants added to vaccines make it possible to improve the immune response (for inactivated vaccines they are essential to obtain immunity) and to give fewer booster doses to obtain effective immunity. Aluminum salts are the most commonly used adjuvants. Preservatives and stabilizers can also be used.
Regulation and marketing of a vaccine:
A vaccine is developed by following the same clinical trial stages as for the development of a drug.
You can find more information about clinical trials in our Health Magazine article: What is a clinical trial?
Vaccines for COVID-19:
The SARS-CoV-2 pandemic, which has spread worldwide, has prompted industrialists to get involved in the fight against the virus. More than 169 vaccine projects are under development, 26 of which are being tested in humans.
For the moment, two vaccines have been given emergency use authorization by the U.S. Food and Drug Administration (FDA). Both of these vaccines developed are RNA vaccines and are developed and marketed by Pfizer/BioNTech and Moderna. A third vaccine developed by Johnson & Johnson is expected to be authorized soon, but does not use RNA.
What is an RNA vaccine?
The principle of vaccination remains the same, to protect the individual when he or she comes into contact with the coronavirus. The vaccine contains the RNA that facilitates production of the spike protein (present on the surface of the SARS-CoV-2 virus particles). It is this protein that allows the virus to enter our cells and infect them.
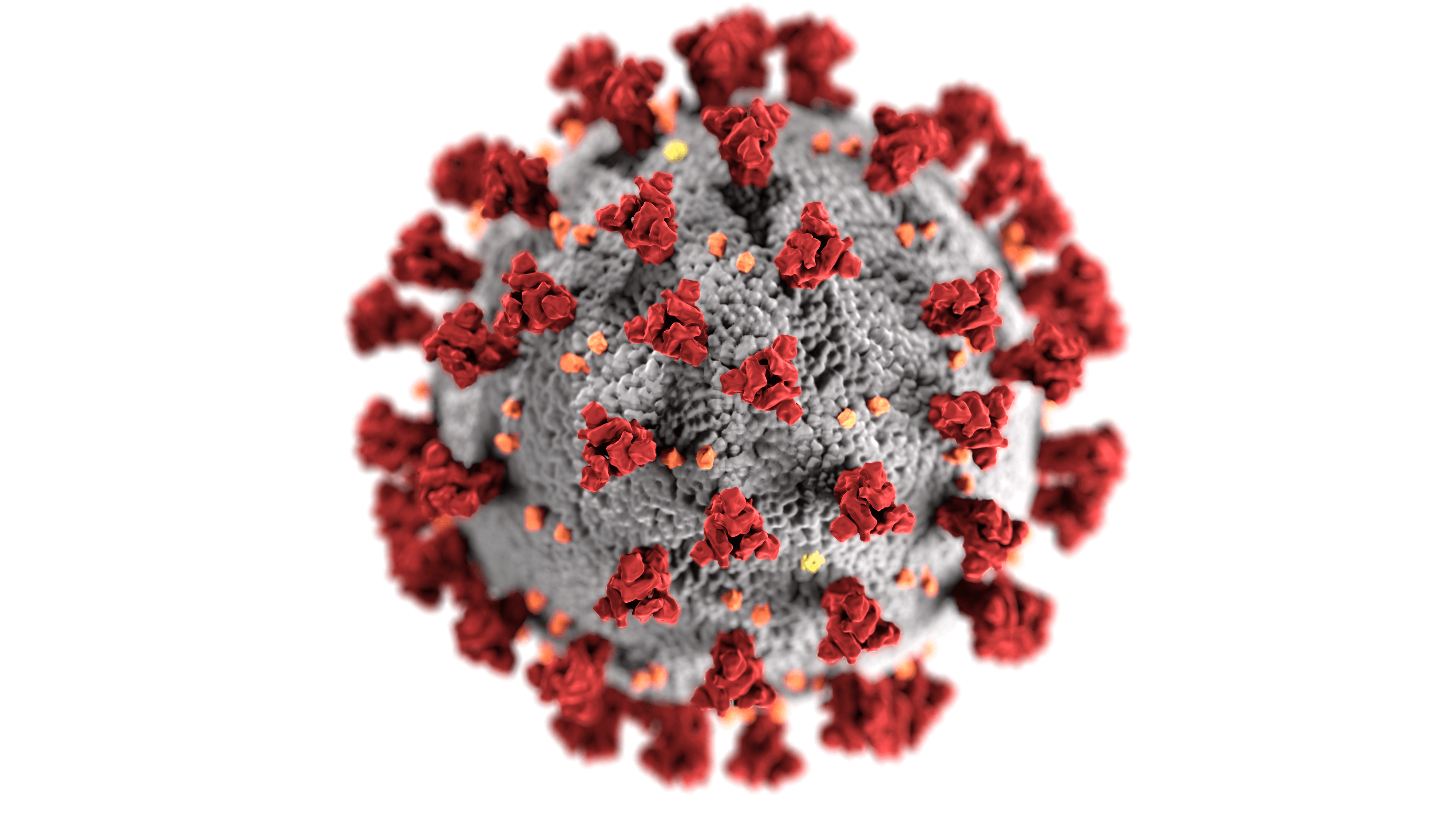
Figure 1. Red spike proteins on the SARS-CoV-2 virion
Source: CDC/ Alissa Eckert, MSMI; Dan Higgins, MAMS
By injecting the RNA of this spike protein, the cells located at the injection site will then be able to produce it themselves, leading to an immune response and therefore the individual's protection against the coronavirus.
The fragility of these RNA proteins imposes storage constraints for the vaccine, in particular a very low temperature (-70°C for the Pfizer/BioNTech vaccine and -20°C for the Moderna vaccine).
Separating the true from the false about RNA vaccines:
These vaccines are faster to produce than vaccines used for other diseases. There are fewer work constraints compared to attenuated or inactivated vaccines and RNA molecules are simpler than DNA molecules to synthesize.
Neither of these two vaccines contains an adjuvant, which explains their fragility, and this is what will allow for them to be better tolerated by individuals.
RNA vaccines do not enter the nucleus of our cells and therefore never come into contact with our genetic material. There is therefore no reason to worry about it modifying our DNA and passing it on to our offspring.
Vaccination program in the US
Because the supply of the COVID vaccine is expected to be limited at first, the CDC has provided recommendations for federal, state, and local governments about who should be vaccinated in priority, following Advisory Committee on Immunization Practices (ACIP) advice.
The CDC recommends that the COVID-19 vaccine supplies be allocated to the following groups in phases:
- Phase 1a:
- Healthcare personnel
- Residents of long-term care facilities
- Phase 1b:
- Frontline essential workers - firefighters, police officers, corrections officers, food and agricultural works, USPS workers, manufacturing workers, grocery store workers, public transit workers, and those who work in the education sector (teachers, support staff, daycare workers
- People aged 75+ - People in this age group who are also residents of long-term care facilities should be offered vaccination in Phase 1a
- Phase 1c:
- People aged 65-74 - People in this age group who are also residents of long-term care facilities should be offered vaccination in Phase 1a
- People aged 16—64 years with underlying medical conditions which increase the risk of serious, life-threatening complications from COVID-19. You can find a list of underlying medical conditions the CDC considers to lead to an increased risk of severe illness from COVID-19 here: People with Certain Medical Conditions
- Other essential workers - those who work in transportation and logistics, food service, housing construction and finance, information technology, communications, energy, law, media, public safety, and public health.
As the vaccine supply increases, vaccination recommendations will expand to include more groups.
While the CDC makes recommendations for who should be offered COVID-19 vaccine first, each state has its own plan for deciding who will be vaccinated first and how they can receive vaccines. Make sure to consult your local health department for more information on COVID-19 vaccination in your area.
Opinions of patients with chronic illnesses:
In order to better understand how patients would react to the arrival of the COVID-19 vaccines, Carenity conducted a survey covering the period from December 3, 2020 to January 18, 2021.
The aim of the survey was to get an idea of how patients felt about coronavirus vaccination.
The participants in this study were of different nationalities:

France | United Kingdom | United States
4784 responses were collected. The graph below shows the distribution of responses according to the participant's country.
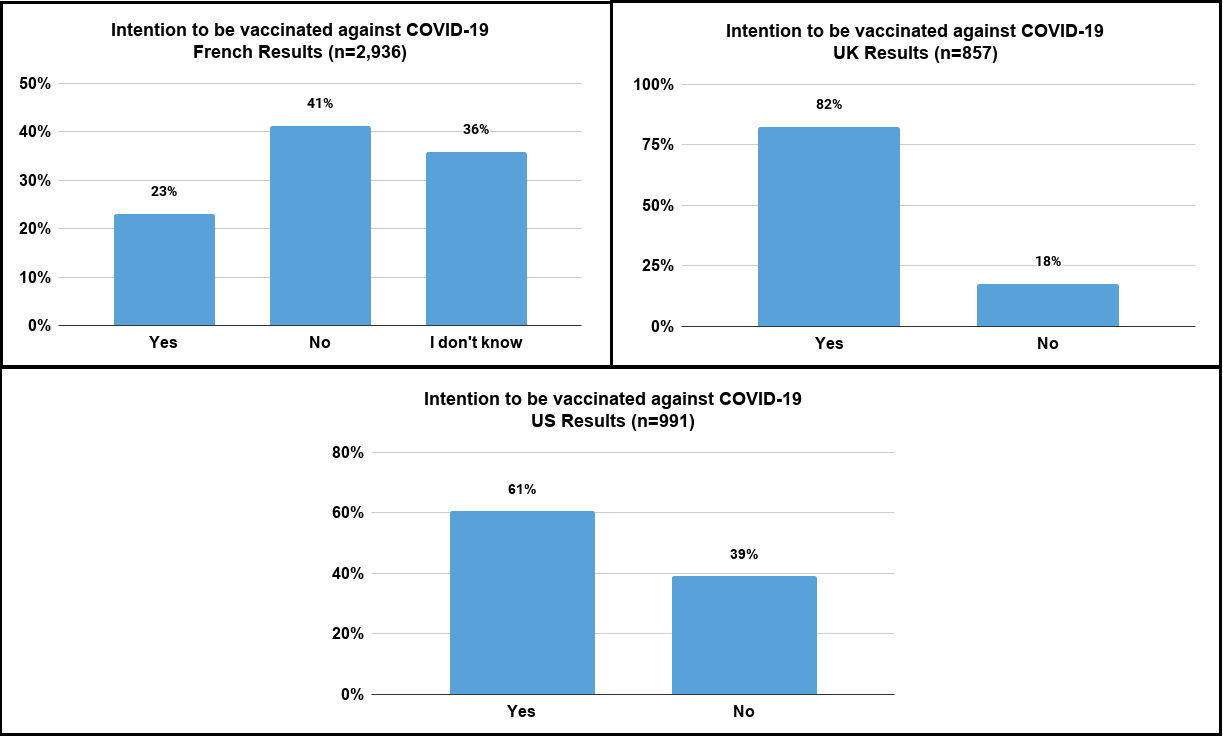
The French are the patients who responded the most to survey with 2,936 responses, followed by the Americans with 991 responses, and then UK members with 857 responses.
![]() Just over 40% of French people do not wish to be vaccinated.
Just over 40% of French people do not wish to be vaccinated.
![]() Only ¼ of French respondents wish to be vaccinated.
Only ¼ of French respondents wish to be vaccinated.
Some respondents are waiting to get more information through their doctor, as is the case for this French respondent:
“As I have always trusted my pulmonologist, I will consult him for his opinion because I know he won't take the decision lightly. I believe he'll have more information than I could find on my own to help me make my decision on if I'll get the vaccine or not."
Other respondents were surprised by the speed of vaccine development compared to conventional vaccines. This contributes to the large percentage of people who do not wish to be vaccinated.
As for the responses from our members in the United Kingdom:
![]() A large majority with more than 80% of respondents in the UK wish to be vaccinated.
A large majority with more than 80% of respondents in the UK wish to be vaccinated.
![]() Less than 20% of UK respondents do not wish to be vaccinated.
Less than 20% of UK respondents do not wish to be vaccinated.
“I intend getting the vaccine if it means me getting my freedom to go out and about again because without it there is a fear of going out,” expressed a respondent in the United Kingdom.
As regards patients living in the United States, the answers are more divided:
![]() Around 60% of American patients wish to be vaccinated,
Around 60% of American patients wish to be vaccinated,
![]() and around 40% of patients who do not wish to be vaccinated.
and around 40% of patients who do not wish to be vaccinated.
The United States is the country with the most cases of coronavirus in the world today. Patient-to-patient discussions show that some patients who have already had COVID-19 are more inclined to be vaccinated.
One respondent wrote: “I would take the vaccine. I want to be ready when things are open again. I want to see family and friends. I want to see my daughter and granddaughter. It's tough watching the news and hearing more people have lost their lives."
COVID-19 vaccination and autoimmune and autoinflammatory diseases:
Many patients with chronic conditions are wondering whether they will be able to receive the SARS-CoV-2 vaccine and whether they are among the priority groups for vaccination. This is the case for patients living with autoimmune or autoinflammatory diseases.
Patients with autoimmune and autoinflammatory diseases such as lupus are at risk of complications related to SARS-CoV-2. Most of these patients are undergoing immunosuppressive treatment, meaning that their immune response is impaired greatly. Vaccination appears to be a good way to prevent coronavirus-related complications in these populations. The two RNA vaccines currently on the market are not contraindicated in patients suffering from autoimmune or autoinflammatory conditions. Follow-up in these patients will need to be stepped up in view of the limited scientific data available.
It is also important to consider each illness and treatment individually. For example, patients on rituximab (RITUXAN®) will have a very low number of B cells. B cells are the cells that produce antibodies and are therefore responsible for immunity following a vaccine injection. Other mechanisms of immunity also play a role in protecting against coronavirus, so vaccination is still indicated in these patients.
Was this article helpful to you?
Share your thoughts and questions with the community in the comments below!
Take care!
Sources:
- Vaccin - OMS
- Quels sont les différents types de vaccins ? - Vaccination Info Service
- Composition des vaccins - Vaccination Info Service
- Les vaccins à ARNm susceptibles de modifier notre génome, vraiment ? - Inserm
- Advisory Committee on Immunization Practices (ACIP) - CDC
- People with Certain Medical Conditions - CDC
- Health Department Directories - CDC
Comments
You will also like
What are the dangers associated with the over-the-counter sale of certain medicines?
Dec 19, 2020 • 6 comments

 Facebook
Facebook Twitter
Twitter